The science of Hanuman’s flight
While Meghnath is busy learning more advanced sciences, Hanuman is flying around with his Air Molecule friends.

Illustration: Sahil Upalekar
Hanuman: Hey Molecules, whatever took place while I was chasing the sun seemed like a dream. But now, I slowly remember experiencing several bizarre things.
Air molecules: Like what, Hanuman?
Hanuman: In the beginning, till we reached the rainy clouds. everything seemed fined. But after flying a few kilometres past them, it was freezing. Why?
Air molecules: To understand that, you will need to learn about gravity, air density, altitude, pressure and temperature.
Hanuman: Woah! That seems like a lot of science!
Air molecules: Everything you experienced at that time is science.
Hanuman: Fine. As long as you don’t put me to sleep…
Air molecules: Ha ha! Don’t worry, we will explain it to you in a way that you will understand. Now, what do you know of gravity?
Hanuman: Anything with mass will have some pulling force. For planets like Earth, we call this force as gravity, which tries to pull everything towards its centre.
Air molecules: Exactly! Basically, everything that exists on Earth is being pulled towards the centre of Earth. The closer you are, the more gravity you experience.
Hanuman: Next, what is density?
Air molecules: It is simply the number of molecules packed within a certain area; be it in air, a solid or liquid. Gases are less dense than solids and liquids because their molecules are far apart.
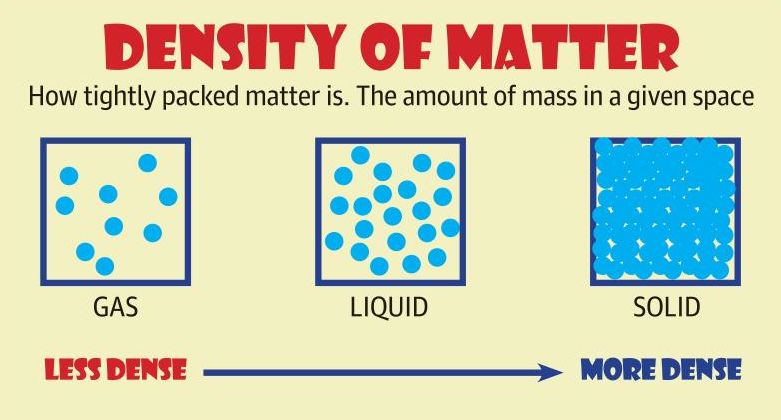
Air molecules:With regards to air density, there are more air molecules closer to the ground. Do you know why?
Hanuman: Because the pull of gravity is more?
Air molecules: That’s right! Now, let’s move on to temperature…
Hanuman: You mean, heat?
Air molecules: Temperature and heat are different. Heat is the transfer of thermal energy between molecules and measured in joules. Temperature is the average kinetic energy of molecules and is measured in Celsius, Kelvin or Fahrenheit.
Hanuman: What is kinetic energy?
Air molecules: Kinetic energy is the energy that a molecule possesses due to its motion. Don’t confuse yourself. Just remember: kinetic means movement.
Hanuman: Okay. Let me see if I understand. When we are closer to Earth, there is more gravity; due to this, there is more density of air molecules; due to more density, there is more kinetic energy, and more kinetic energy means higher temperature. Am I right?
Air molecules: You are super smart, Hanuman! So, what do you think happens as we move away from the ground?
Hanuman: Gravity reduces…
Air molecules: Exactly. As gravity reduces, the density of air molecules decreases, thus kinetic energy decreases, lowering the temperature.
Hanuman: So that is why we go to hill stations. The temperature is less there!
Air molecules: Shall I teach you a trick? It will be useful when you fly and reach great heights.
Hanuman: What’s that?
Air molecules: As you move up every 1 km, the temperature drops by 5.45 to 6 degrees.
Hanuman: In that case, if the ground temperature is 30°C degrees, when I fly up 5 km, it becomes zero?
Air molecules: That’s right. But this calculation works only until 20 km. After that, it’s different; as we cross the ozone layer, and the sun’s direct high energy radiation starts hitting the molecules.
Hanuman: Oh! I will remember that.
Air molecules: The concept of pressure is also similar. As there are more molecules closer to the ground, there is more pressure.
Hanuman: Pressure is force per area, right?
Air molecules: Yes. More the number of molecules and movement, the more the pressure. Lesser the air molecules, the lesser the pressure.
Hanuman: Wow! It took my guru a whole year to make me learn all this! You are great teachers, Molecules.
Air molecules: Thank you! If you start looking at the science around you practically, it becomes more fun and magical.
Hanuman: I would love to learn more!
The author is the founder and CEO of Vaayusastra Aerospace, an IIT-Madras incubated ed-tech startup that offers Air Science workshops for children between five and 14 years.
How well have you read the text?
Based on your reading of this article, can you answer the following true or false questions?